The Trending With Impact series highlights Aging (Aging-US) publications that attract higher visibility among readers around the world online, in the news, and on social media—beyond normal readership levels. Look for future science news about the latest trending publications here, and at Aging-US.com.
—
Many aging-associated neurodegenerative disorders, including Alzheimer’s disease, involve the aggregation of abnormal tau in nerve cells (neurons). Normally, tau proteins function to stabilize microtubules in the brain. Tauopathy occurs when tau proteins become misfolded and misshapen (which turns tau into toxic tau). They then continue to proliferate and bind to each other, forming tau oligomers. These tau oligomers are more toxic and have a greater potential to spread tau pathology. Before toxic tau snowballs into neurodegenerative disorders, the events that lead up to abnormal tau have remained elusive to researchers.
“While the association between tau levels and energy metabolism is established, it is not clear whether mitochondrial dysfunction is an early pathological feature of high levels of tau or a consequence of its excessive formation of protein aggregates.”
Previous studies have demonstrated an association between tau levels and mitochondrial metabolism, however, determining which one proceeds the other has yet to be fully illuminated. Shedding light on this subject, researchers—from the University of Copenhagen, National and Kapodistrian University of Athens and the National Institutes of Health’s National Institute on Aging—used a Caenorhabditis elegans (C. elegans; roundworm/nematode) model of tau to examine mitochondrial changes over time. Their paper was chosen as the cover of Aging (Aging-US) Volume 13, Issue 21, published in November of 2021 and entitled, “Alteration of mitochondrial homeostasis is an early event in a C. elegans model of human tauopathy”.
The Study
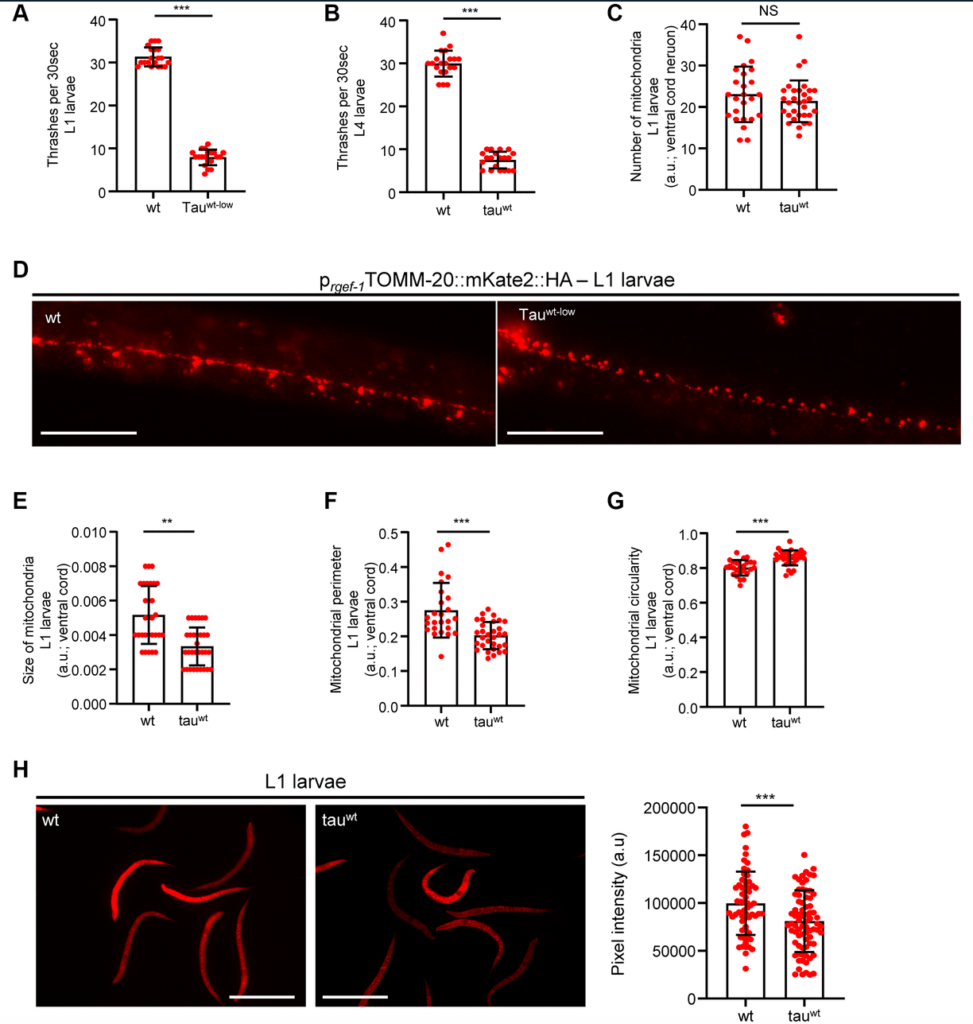
“Here, we utilized transgenic nematodes expressing the full length of wild type tau in neuronal cells and monitored mitochondrial morphology alterations over time.”
To investigate the impact of tau on mitochondrial activity, neuronal function and organismal physiology, the researchers selected and cultured an already characterized nematode strain that expresses the full length of wild type human tau protein. They compared wild type nematodes with tau-expressing nematodes (at various ages) over time using a thrashing assay, mitochondrial imaging, worm tracking software, and western blot analysis. Calcium deregulation was also examined to determine whether or not it is implicated in the impairment of mitochondrial activity in the tau-expressing nematodes. They found that chelating calcium led to restored mitochondrial activity and suggested a link between mitochondrial damage, calcium homeostasis and neuronal impairment in this nematode model.
Conclusion
“Our findings suggest that defective mitochondrial function is an early pathogenic event of tauopathies, taking place before tau aggregation and undermining neuronal homeostasis and organismal fitness.”
The researchers were forthcoming about limitations in their study, given the differences between human and nematode biology and pathology. Nevertheless, they found evidence that, in this nematode tauopathy model, neurotoxicity depends on protein alterations and mitochondrial dysfunction. Mitochondrial dysfunction takes place before high levels of tau are detected. Tau mutations may also modulate calcium homeostasis by influencing the main cellular storage sites—the endoplasmic reticulum and mitochondria.
“Investigating the tight interplay between tau oligomers and energy metabolism will enlighten new avenues for therapeutic strategies to slow or halt the progression of dementia-related diseases such as AD [Alzheimer’s disease].”
Click here to read the full priority research paper published by Aging (Aging-US).
WATCH: AGING VIDEOS ON LABTUBE
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].

