The Trending with Impact series highlights Aging (Aging-US) publications that attract higher visibility among readers around the world online, in the news, and on social media—beyond normal readership levels. Look for future science news about the latest trending publications here, and at Aging-US.com.
—
Compounding evidence from experimental, epidemiological and clinical studies indicates that vascular dysfunction, including cerebral amyloid plaque formation and reduced cerebral blood flow (CBF), plays an important role in the cognitive decline associated with aging and age-related diseases. Recently, hyperbaric oxygen therapy (HBOT) was shown to improve cognitive performance in animal models of Alzheimer’s disease (AD) and in human patients. However, previous research has not directly shown how, or if, HBOT mitigates cerebrovascular dysfunction in AD.
“Therefore, we investigated the effects of HBOT on CBF and cognitive decline in the 5XFAD mouse model of AD that presents aggressive accumulation of amyloid load [40], cerebrovascular abnormalities [15, 41, 42] and cognitive impairment [40], as well as in elderly individuals suffering from significant memory loss.”
Researchers from Tel Aviv University and Shamir (Assaf Harofeh) Medical Center conducted a new research study in an effort to better understand the underlying mechanisms of HBOT-mediated effects. They authored a trending research paper, published by Aging (Aging-US) in August 2021, entitled, “Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer’s disease mouse model and in elderly patients.”
The Study
In this study, the researchers observed the effects of HBOT in six human participants and in the 5XFAD mouse model of AD. The study mice were placed in a chamber and exposed to 100% oxygen under two ATA (twice the atmospheric pressure at sea level) for 60 minutes per day and five days per week over the course of four consecutive weeks. The control mice remained under normal atmospheric oxygen conditions. To assess the results of HBOT in the study mice, researchers used behavioral testing, the Y-maze test, trace fear conditioning, biochemical and histological analyses, immunochemistry, hypoxyprobe staining, immunoblotting, Aβ enzyme-linked immunosorbent assay (ELISA), cranial window generation, and two-photon imaging.
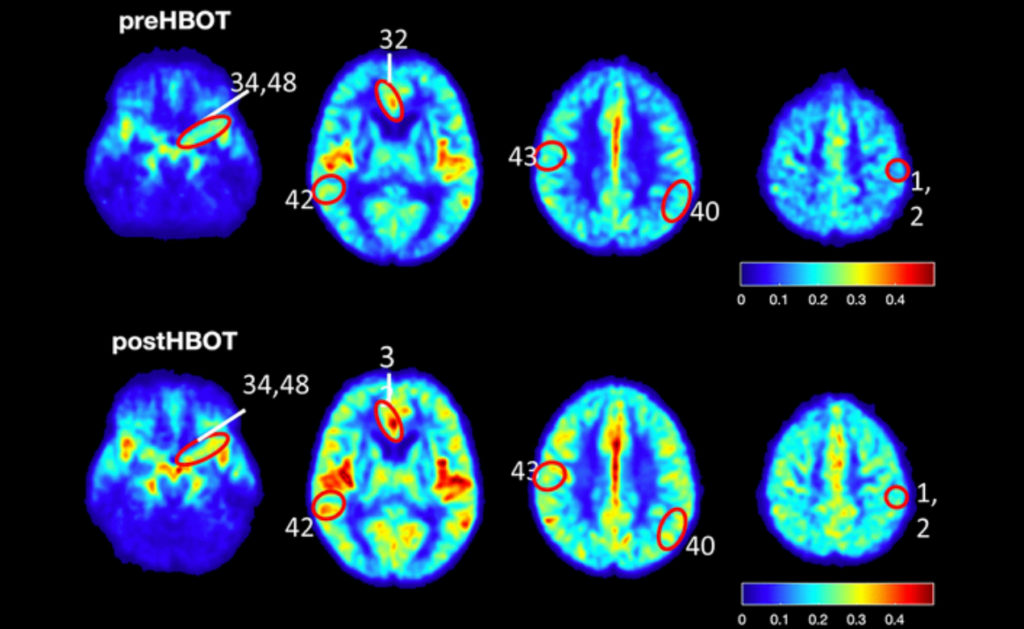
Six human subjects (five male and one female) aged 64 years and older, who were living independently but had significant memory decline, were also administered HBOT. The researchers enlisted a detailed exclusion criteria to select their candidates. HBOT sessions were scheduled for the human subjects for 90 minutes per day (with five minute air breaks every 20 minutes) at five days per week over the course of three consecutive months. To assess the ability of HBOT to change CBF and affect cognitive function in the human subjects, the researchers used cognitive assessments, MRI scans and statistical analysis.
Results
In 5XFAD mouse models of Alzheimer’s disease, HBOT reduced amyloid load in mice by reducing newly-formed plaques and decreasing the volume of existing plaques. HBOT alleviated the reduction of vessel diameter and increased blood flow and arteriolar lumen size. The researchers found that HBOT reduced hypoxia and hypoxia inducible factor-1 levels and also improved 5XFAD mouse performance in behavioral tasks. Lastly, the researchers demonstrated that HBOT increases cerebral blood flow and improves cognitive performances in elderly patients.
Conclusion
“Furthermore, by tracking single plaques in vivo over weeks, we show for the first time that HBOT reduces the volume of pre-existing plaques and the appearance of newly-formed plaques.”
The researchers were forthcoming about limitations of their study. They note that a relatively small size human cohort was enrolled, and encourage future studies to use a larger human cohort to verify their findings. Their findings further demonstrate the connection between vascular pathology and neurodegeneration. Moreover, the researchers demonstrated the ability of HBOT to improve hypoxia-related neurological conditions, such as Alzheimer’s disease.
“Together with our findings using an AD mouse model and the similar effects observed following HBO treatment of stroke and TBI patients, we suggest that HBOT mediates structural changes in blood vessels that increase CBF, reduce brain hypoxia and improve cognitive performance.”
Click here to read the full research paper, published by Aging.
WATCH: AGING VIDEOS ON LABTUBE
—
Aging is an open-access journal that publishes research papers monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].